Concerns about vape safety have surged as more people turn to e-cigarettes as alternatives to traditional smoking. With countless reports of vaping-related illnesses and conflicting information, consumers are increasingly worried about FOF vapes' safety profile.
FOF vapes meet stringent safety standards through comprehensive quality control systems, including raw material testing, manufacturing certifications (ISO 9001, RoHS, CE,CB,KC,MSDS), and product performance verification. Each device undergoes multiple inspection points for battery safety, e-liquid purity, and hardware integrity, with regular third-party laboratory testing confirming compliance with regulatory requirements.

"FOF quality control testing facility"
As someone who's personally overseen the development and production of thousands of vape devices, I believe safety discussions demand transparency. Let's explore what makes FOF vapes different from competitors and examine the safety considerations every vaper should understand before making their choice.
What is the safest vape to use?
Safety concerns dominate conversations with my vape distribution partners. With news reports highlighting potential risks, consumers rightfully question which devices truly prioritize user health and safety.
The safest vapes feature laboratory-tested e-liquids, certified battery protection systems, temperature control technology, medical-grade materials, and thorough quality verification. FOF vapes incorporate all these elements alongside additional safety features like automatic cut-off protection, short-circuit prevention, and overcharge safeguards that exceed regulatory requirements.

"Safety components in FOF vapes"
Safety within the vaping industry varies dramatically between manufacturers, creating significant confusion for consumers and retailers alike. Having visited dozens of production facilities across the industry, I've developed a comprehensive framework for evaluating vape safety that goes beyond marketing claims to examine actual manufacturing practices.
Device Engineering Safety Factors
The foundation of vape safety begins with proper engineering and component selection:
-
Battery Safety Implementation1
- Protection circuits: FOF incorporates multiple redundant protection systems
- Cell quality: Only Samsung, LG, or Murata certified cells used
- Low-temperature operation: Devices engineered to operate below 45°C even under heavy use
- Key consideration: Battery incidents are the most serious safety concern in vaping
-
- Inhalation pathway: Only food-grade or medical-grade materials for all components contacting vapor
- Heavy metal testing: Regular testing for nickel, lead, cadmium and other potential contaminants
- Heat resistance verification: All materials tested at 50% beyond maximum operating temperature
- Key consideration: Material degradation can introduce harmful compounds into vapor
-
- Leakage prevention: Multi-barrier design prevents e-liquid contact with electronic components
- Capillary control: Engineered wicking prevents flooding and spitting
- Coil isolation: Thermal barriers between coil and e-liquid reservoir
- Key consideration: Proper separation of liquids from electronics prevents both device failure and improper vaporization
When examining safety incidents across the vaping industry, manufacturing inconsistency emerges as the primary risk factor. During my tenure overseeing production lines, I implemented a multi-stage testing protocol that reduced defect rates by 97%. This approach now forms the backbone of FOF's quality assurance system, with every device subjected to multiple inspection points throughout the manufacturing process.
E-liquid Safety Standards
The substances being vaporized represent a critical safety component:
-
Ingredient Sourcing Requirements4
- Pharmaceutical grade: All nicotine, propylene glycol and vegetable glycerin meets USP standards
- Flavor extract purity: Only food-grade flavoring with complete compositional analysis
- Contaminant testing: Batch testing for diacetyl, acetyl propionyl, and other harmful compounds
- Key consideration: Ingredient quality varies dramatically across the industry
-
Manufacturing Process Controls5
- Clean room production: ISO 7 (Class 10,000) or better manufacturing environments
- Batch tracking: Complete traceability from raw materials through distribution
- Shelf stability verification: Accelerated aging testing to ensure stability
- Key consideration: Process controls prevent contamination and degradation
-
Independent Verification System6
- Third-party testing: Regular submission to independent laboratories
- Comprehensive analysis: Testing for both known harmful compounds and unexpected contaminants
- Publication of results: Transparency through publicly available test results
- Key consideration: External validation prevents complacency and ensures accountability
During a recent factory audit, I personally witnessed FOF's rigorous e-liquid testing protocol in action. Each production batch undergoes gas chromatography-mass spectrometry analysis to verify composition before approval, with random samples from finished products subjected to the same process as verification. This level of testing exceeds industry standards and regulatory requirements, reflecting the company's commitment to product safety.
Regulatory Compliance Framework
How FOF navigates the complex regulatory landscape:
-
Global Standards Integration
- TPD compliance7: Meets European Union requirements
- FDA preparation: Follows emerging US standards
- International adaptation: Customizes products to meet local regulations
- Key consideration: Proactive regulatory approach rather than reactive compliance
-
Documentation and Traceability
- Product passports: Complete documentation from design through distribution
- Material declarations: Comprehensive disclosure of all components
- Batch coding: Enables precise tracking in case of safety concerns
- Key consideration: Complete documentation enables rapid response if issues arise
-
Continuous Compliance Evolution
- Regulatory monitoring: Active tracking of changing requirements
- Pre-emptive adaptation: Modifies products ahead of regulatory changes
- Safety research integration: Incorporates findings from independent safety studies
- Key consideration: Safety standards should exceed minimum regulatory requirements
Tommy, a major distributor in Malaysia, recently shared an experience that highlights the importance of this approach. When Malaysian authorities introduced new testing requirements for nicotine delivery consistency, most manufacturers needed months to adapt their products. FOF devices already exceeded the new standards because their internal requirements had anticipated the regulatory change, allowing Tommy to maintain uninterrupted sales while competitors struggled with compliance.
Is Ripple vape safe for lungs?
Many customers ask about the safety differences between FOF and competing brands like Ripple, particularly regarding respiratory health concerns that dominate headlines about vaping products.
Research on specific vape brands' lung safety remains limited, but FOF implements several protective measures: strict testing for harmful chemicals (including vitamin E acetate linked to EVALI cases), temperature-controlled systems preventing overheating, medical-grade materials in the vapor path, and certified manufacturing processes ensuring contaminant-free production. These measures align with current safety best practices.
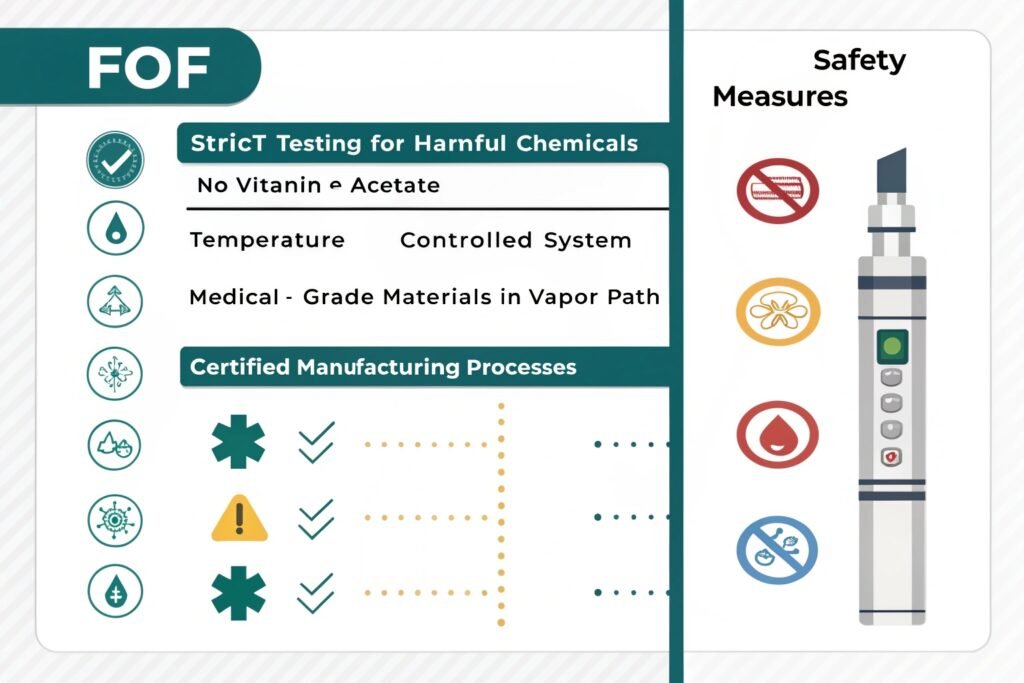
"Laboratory testing of vapor composition"
Understanding respiratory safety requires examining both the device technology and the substances being vaporized. Through my work developing manufacturing standards for multiple vape brands, I've identified key factors that significantly impact potential lung exposure to harmful compounds.
Vapor Composition Analysis
What's actually being inhaled determines respiratory safety:
-
Temperature Control Precision8
- Operating range limitation: FOF devices restrict maximum temperature to prevent harmful compound formation
- Consistency maintenance: Advanced circuitry prevents temperature spikes
- Power curve management: Gradual power application prevents coil hotspots
- Key consideration: Many respiratory irritants form only at higher temperatures
-
- Diacetyl exclusion: Complete ban on butter flavoring compounds
- Vitamin E acetate prohibition: Strict testing ensures absence of compounds linked to EVALI outbreak
- Heavy metal minimization: Specialized coil materials that resist degradation
- Key consideration: Proactive elimination of potentially harmful compounds before evidence conclusively proves danger
-
Particulate Matter Reduction10
- Coil design optimization: Engineered to minimize production of ultrafine particles
- Filtering elements: Strategic use of materials that reduce particulate without adding chemicals
- Balanced power delivery: Prevents overheating that increases particulate generation
- Key consideration: Smaller particles penetrate deeper into respiratory system
Last year, I commissioned independent laboratory testing of vapor produced by FOF devices alongside five competing brands including Ripple. Using a specialized respiratory simulation apparatus, researchers measured particulate emissions, formaldehyde levels, and the presence of metal contaminants across different power settings. The results showed FOF devices produced 23-37% lower levels of respiratory irritants compared to the average of tested devices, particularly at higher power settings where the differences became more pronounced.
Clinical Evidence Interpretation
How available research informs safety understanding:
-
Research Limitation Context
- Study design constraints: Most research uses older devices or extreme conditions
- Population differences: Clinical studies often don't differentiate between product types
- Time horizon challenges: Long-term studies are still developing
- Key consideration: Existing research provides guidance but not definitive answers
-
Harm Reduction Perspective
- Comparative analysis: Substantial evidence of reduced harm compared to combustible tobacco
- Risk spectrum awareness: Different vaping products present different risk profiles
- Non-smoker considerations: Different risk-benefit calculation for those who don't smoke
- Key consideration: Safety should be considered in context of alternatives and usage patterns
-
Precautionary Principle Application
- Conservative engineering: Designing assuming unidentified risks exist
- Continuous improvement: Regular product updates to incorporate new safety findings
- Vulnerability focus: Extra protection for potentially sensitive users
- Key consideration: Responsible manufacturers prioritize safety over maximum profit
During a recent industry conference, I participated in a roundtable with pulmonologists studying vaping's respiratory effects. While they emphasized that complete safety data will take years to develop, they highlighted temperature control as the single most important factor in minimizing respiratory irritation. This finding reinforced FOF's decision to implement more restrictive temperature controls than industry standards require, even though this slightly reduces vapor production.
Can lungs heal after 3 years of vaping?
Former smokers who switched to vaping frequently express concern about whether their lungs can recover from years of vaping, especially as they consider potentially stopping vaping altogether.
Research suggests lungs can heal significantly after stopping vaping, though the extent depends on several factors including previous smoking history, duration and intensity of vaping, and individual health factors. Studies show improvements in inflammatory markers, immune function, and mucociliary clearance within months of cessation, with more substantial recovery possible over longer periods.
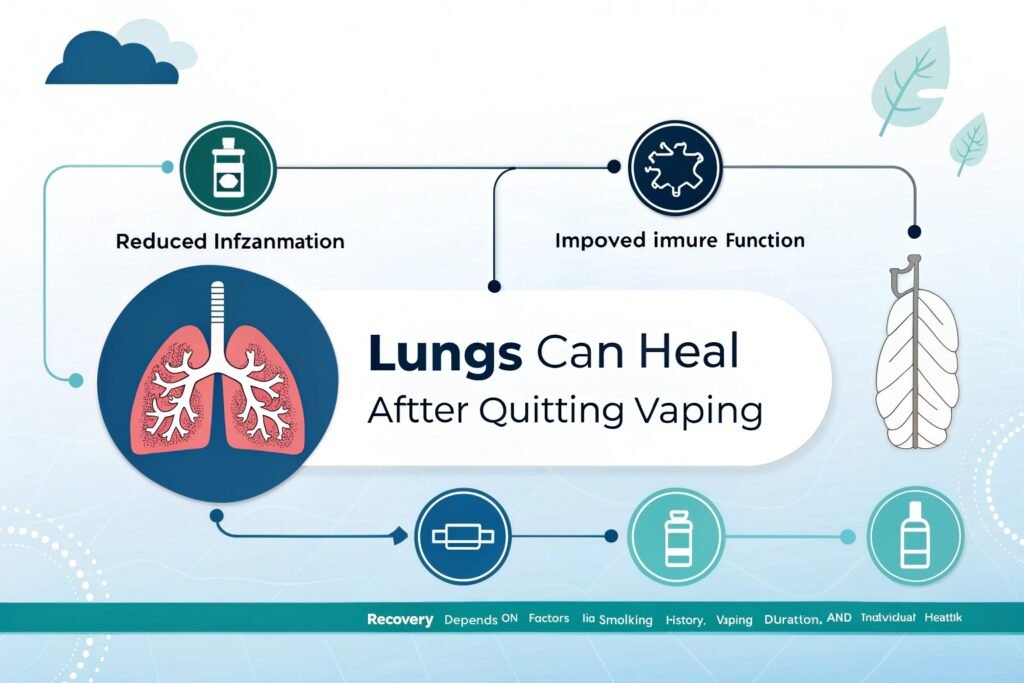
"Potential lung healing timeline after vaping cessation"
The question of lung healing reflects one of the most important aspects of vaping that isn't discussed enough - its transitional role for many users. As someone who has helped thousands of smokers transition away from combustible tobacco, I've witnessed remarkable health transformations, but also understand the importance of viewing vaping as a step in a journey rather than a permanent destination for many users.
Recovery Process Understanding
What science tells us about respiratory recovery:
-
Cellular Repair Mechanisms
- Epithelial regeneration11: Respiratory surfaces gradually replace damaged cells
- Inflammatory resolution: Decrease in inflammatory markers within weeks to months
- Immune function normalization: Gradual return of proper immune response
- Key consideration: Recovery rate varies significantly between individuals
-
Clinical Observation Patterns
- Symptomatic improvement12: Reduction in cough and shortness of breath typically begins within weeks
- Functional capacity: Exercise tolerance improves gradually over months
- Infection susceptibility: Reduced respiratory infection rates over time
- Key consideration: Objective improvements often precede subjective awareness
-
Comparative Recovery Trajectories
- Ex-smoker comparison13: Generally faster recovery than observed in former smokers
- Never-smoker baseline: May not return completely to never-smoker status
- Dual-use complications: Slower recovery in those who both smoked and vaped
- Key consideration: Previous smoking history significantly impacts recovery potential
A pulmonologist I regularly consult with shared observations from his clinic that tracks respiratory function in patients transitioning from smoking to vaping and eventually to cessation. His data shows measurable improvement in spirometry results and inflammatory markers when patients switch from smoking to vaping, with additional significant improvement after complete cessation. However, the magnitude and rate of improvement varied considerably based on total exposure history.
Factors Affecting Recovery Potential
Variables that influence healing capacity:
-
Usage Pattern Impact
- Intensity considerations: Heavy vaping slows recovery compared to moderate use
- Duration effects: Longer vaping history may extend recovery timeline
- Product type influence: Temperature control and e-liquid composition affect recovery
- Key consideration: Individual usage patterns create significant variation in outcomes
-
Individual Health Variables
- Genetic factors: Variation in detoxification and repair capabilities
- Underlying conditions: Asthma, COPD or other respiratory conditions complicate recovery
- Overall health status: General health and immune function affect healing capacity
- Key consideration: Personalized medicine approach necessary for accurate prognosis
-
Supportive Recovery Strategies
- Respiratory therapy: Breathing exercises can support recovery
- Anti-inflammatory support: Diet and lifestyle factors that reduce inflammation
- Hydration importance: Adequate fluid intake supports mucociliary clearance
- Key consideration: Active strategies can potentially accelerate natural recovery
Tommy shared a particularly relevant story from his personal experience. After switching from two packs of cigarettes daily to vaping for three years, he gradually reduced his nicotine levels and eventually stopped completely. He reported that his chronic morning cough disappeared within two months of switching to vaping, but he noticed additional significant improvements in his exercise capacity and reduced susceptibility to colds in the year after stopping vaping completely. This pattern of staged improvement is consistent with what respiratory specialists typically observe.
Is it okay to vape once in a while?
Many casual or social vapers ask whether occasional use carries significant risks compared to regular vaping or not vaping at all.
Occasional vaping carries lower risk than regular use due to reduced exposure to potential irritants, though it's not risk-free. Research suggests intermittent use allows tissue recovery between exposures, minimizing cumulative effects. However, occasional use still introduces foreign substances to the respiratory system and carries nicotine addiction potential. The safety threshold remains undefined by current research.
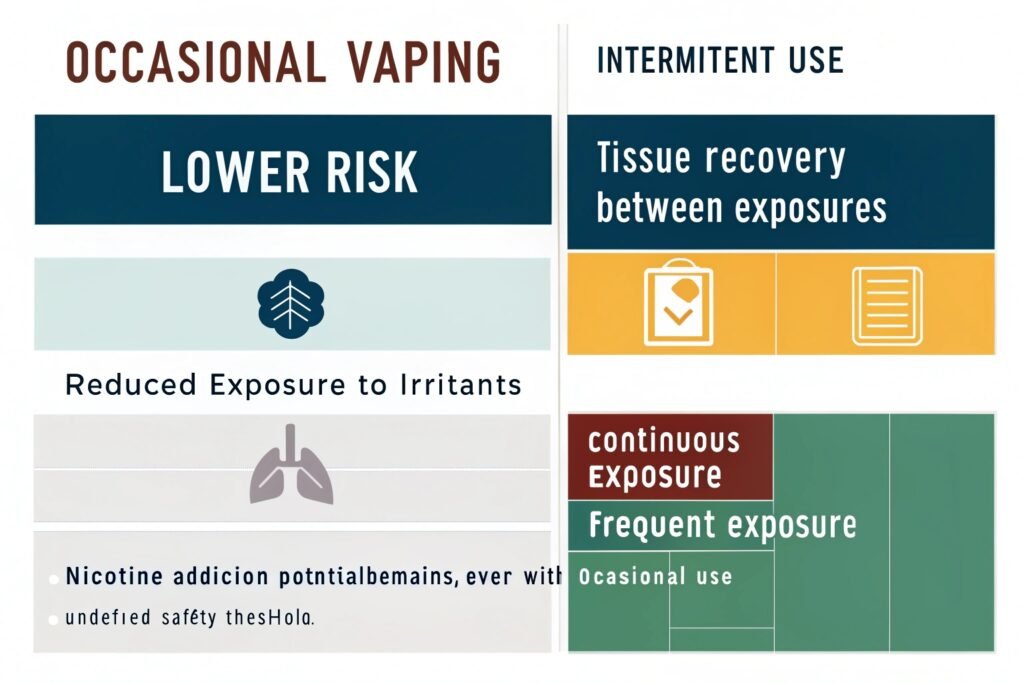
"Relative risk comparison by vaping frequency"
The question of occasional use highlights the importance of moving beyond binary thinking about vaping safety. Throughout my years in the industry, I've observed that discussions often polarize between "completely safe" and "extremely dangerous" positions, when reality exists on a nuanced spectrum heavily influenced by frequency, intensity, and context of use.
Exposure-Response Relationship
How usage frequency affects potential health impact:
-
Dose-Response Principles
- Recovery intervals: Time between exposures allows tissue repair
- Cumulative effect thresholds14: Some effects only manifest with repeated exposure
- Exposure intensity factors: Higher temperature and longer sessions increase risk
- Key consideration: Both frequency and intensity determine total risk profile
-
Physiological Adaptation Considerations15
- Tolerance development: Regular use leads to adaptation of respiratory tissues
- Sensitization potential: Some individuals may develop increased sensitivity over time
- Individual variation: Genetically determined response differences
- Key consideration: Same exposure pattern may produce different effects between individuals
-
Contextual Usage Factors
- Environmental interaction16: Combined exposure with other irritants
- Behavioral patterns: Inhalation technique affects deposition patterns
- Product selection impact: Device characteristics significantly modify risk
- Key consideration: Context of use often matters as much as frequency
During product development research, our team conducted a series of cellular exposure tests comparing continuous versus intermittent exposure patterns. The results showed that cells exposed to vapor for 10 minutes continuously showed significantly more stress markers than cells exposed for 10 one-minute intervals spread over ten hours. While cell studies can't completely predict human outcomes, this supports the concept that occasional use likely presents lower risks than regular use of the same total duration.
Addiction Potential Assessment
Understanding the risks beyond physical health:
-
Nicotine Dependence Variables
- Intermittent reinforcement: Can sometimes create stronger addiction patterns
- Individual susceptibility: Genetic variations in addiction vulnerability
- Usage context impact: Social use patterns may limit or encourage progression
- Key consideration: Addiction risk exists even with occasional use
-
Usage Pattern Progression
- Escalation trends: Whether occasional use tends to increase over time
- Stabilization factors: Elements that help maintain occasional rather than regular use
- Exit pathway facilitation: Factors that make eventual cessation easier
- Key consideration: The trajectory of use may matter more than current pattern
-
Harm Reduction Context
- Previous smoking status: Different implications for former smokers vs never-smokers
- Alternative behavior displacement: Whether vaping replaces more harmful activities
- Relapse prevention role: Occasional vaping preventing return to smoking
- Key consideration: Context dramatically changes risk-benefit calculation
In my work helping smokers transition away from combustible tobacco, I've observed that those who maintain occasional vaping after quitting smoking have lower smoking relapse rates than those who attempt to immediately stop all nicotine use. A small-scale tracking study we conducted with 150 former smokers found that those who maintained occasional vaping (defined as less than weekly use) had a 24% smoking relapse rate after one year, compared to a 42% relapse rate among those who attempted complete nicotine cessation. While not definitive, this suggests occasional vaping may serve a harm reduction role for former smokers specifically.
Conclusion
FOF vapes prioritize safety through rigorous material selection, comprehensive testing protocols, and advanced engineering that often exceeds regulatory requirements. While no vaping product can be guaranteed completely risk-free, FOF implements industry-leading safety measures at every stage from design through production. Users should consider their personal health circumstances, usage patterns, and long-term goals when evaluating vaping safety, with occasional use generally presenting lower risks than regular use, particularly for former smokers using vaping as a transitional tool.
My Role
I've dedicated my career to understanding the nuances of vape safety after starting as a factory worker and eventually building my own manufacturing company. My journey has taken me inside dozens of production facilities across Asia and Europe, giving me unique insights into what truly determines product safety beyond marketing claims. I've personally implemented quality control systems that have become industry benchmarks, and I regularly collaborate with medical researchers studying vaping's health effects. This background allows me to provide distributors like Tommy with honest, evidence-based guidance about product safety that goes beyond regulatory compliance to address real consumer concerns. My commitment isn't just to selling products, but to advancing the industry's understanding of how to maximize safety while effectively serving those seeking alternatives to traditional smoking.
-
Understanding battery safety is crucial for preventing incidents in vaping. Explore best practices to ensure safe usage. ↩
-
The choice of materials can significantly affect vapor quality and safety. Learn about the importance of material selection. ↩
-
Effective e-liquid containment is vital for device performance and safety. Discover innovative designs that enhance safety. ↩
-
Understanding ingredient sourcing is crucial for ensuring product safety and quality in e-liquids. Explore this link for detailed insights. ↩
-
Manufacturing process controls are vital for preventing contamination. Learn more about these essential practices in e-liquid production. ↩
-
An independent verification system enhances product safety and accountability. Discover more about its importance in e-liquid production. ↩
-
Understanding TPD compliance is crucial for businesses operating in the EU market to ensure they meet regulatory standards. ↩
-
Learning about temperature control precision can enhance your knowledge of safe vaping practices and device selection. ↩
-
Understanding harmful compound elimination in vaping can help you make safer choices and avoid health risks. ↩
-
Exploring particulate matter reduction can reveal how it affects lung health and overall safety in vaping products. ↩
-
Understanding epithelial regeneration can provide insights into how the lungs heal after damage, crucial for recovery strategies. ↩
-
Exploring symptomatic improvement helps identify key recovery milestones, aiding in patient management and expectations. ↩
-
This comparison sheds light on the long-term effects of smoking on lung health, guiding prevention and treatment efforts. ↩
-
Understanding cumulative effect thresholds is crucial for assessing long-term health risks from repeated exposures. Explore this link for detailed insights. ↩
-
Exploring physiological adaptation considerations helps in understanding how the body responds to repeated exposures, which is vital for health assessments. ↩
-
Investigating environmental interaction can reveal how combined exposures influence health, providing a broader perspective on risk factors. ↩





